Project 1
Peptide Transport and Interactions with Membranes and Extracellular Matrix
(Drs. Greathouse, Ivey, Sakon, Hinton, Koeppe, Schäfer).
X-ray Crystal Structure of Collagen Binding Domain
(Joshua Sakon , Jeff Wilson, Osamu Matsushita)
Our goal is to determine the structure of Collagen Binding Domain (CBD) of Clostridium
collagenase, in order to develop novel drug delivery systems directed at the extracellular
matrix. We have recently solved the structure of CBD to 0.95 Å, revealing a beta-sheet
sandwich fold with an N-terminal alpha-helix extending across the collagen-binding
surface (Figure A). The collagen interaction site consists of conserved tyrosine residues
on the surface of CBD (see arrows in Figure A). Surprisingly, the addition of calcium
causes the N-terminal helix to unwind, resulting in a new beta-strand on the back
face of the protein structure (Figure B). We speculate that calcium could be acting
as a switch for CBD to be activated. Our goal is to use this structural information
to develop new collagen-binding peptides which could deliver drugs to targeted sites
for increased effectiveness.
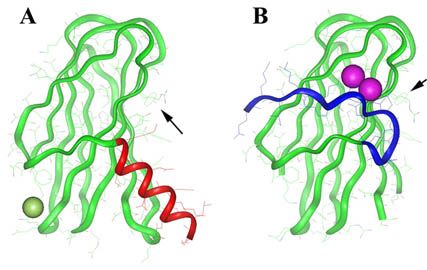
X-ray Crystal Structure of Collagenase CBD with (B) and without (A) Calcium.
Publications
- Jeff Wilson, Osamu Matsushita, *Joshua Sakon, Ca2+ induced switch from helix to sheet triggers collagen binding. American Crystallographic Association Annual Meeting, July 21, 2001.
- *Joshua Sakon, Osamu Matsushita, 0.95 Å structure of collagen binding domain and Ca2+ induced switch. American Crystallographic Association Annual Meeting, July 21, 2001.
|
Gramicidin A Cation Channel |
Cation Transport Across Biological Membranes
Denise Greathouse, Roger Koeppe
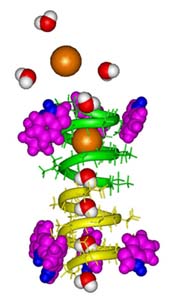
Gramicidin A is a 15-amino acid peptide that forms a dimeric channel that is highly selective for the transport of cations such as sodium across biological membranes (Figure). Gramicidin A has become an important system for addressing fundamental questions about cation transport, a process which plays an essential role in many important biological functions, including nerve transmission in brain and muscle. New protein engineering techniques are being used to reveal how each atom in gramicidin A contributes to the conductance of cations with such high selectivity. The gramicidin A transmembrane "miniprotein" ion channel is stabilized by interfacial tryptophans, four at each side of the membrane (Figure). Our results are providing insight into the specific role of tryptophan in the folding, structure, lipid interactions and function of membrane proteins.
Publications
- *Greathouse, Denise, V., Goforth, Robyn, L., Crawford, Toria, van der Wel, Patrick, and Killian, J. Antoinette, Optimized aminolysis conditions for cleavage of N-protected peptides from solid-phase resins J. of Peptide Research 57 519-527 (2001).
- Jude, A. R., Providence, L.L., Schmutzer, S. E., *Greathouse, D.V., Andersen, O.S., and Koeppe, R.E. II., Peptide backbone chemistry and membrane channel function: Effects of a single amide-to-ester replacement on gramicidin channel structure and function Biochemistry 40 1460-1472 (2001).
- De Planque, M.R.R., Goormaghtigh, E., *Greathouse, D. V., Koeppe, R. E. II, Kruijtzer, J. A. W., Liskamp, R. M. M., de Kruijff, B., and Killian, J. A., Sensitivity of single membrane-spanning a-helical peptides to hydrophobic mismatch with a lipid bilayer: Effects on backbone structure, orientation and extent of membrane incorporation Biochemistry 40, 5000-5010 (2001).